Background: Ibrutinib is a first-in-class Bruton's tyrosine kinase inhibitor (BTKi) that has changed the treatment paradigm for patients with chronic lymphocytic leukemia (CLL) with durable responses and improved overall survival (OS). In the pivotal Phase 3 GLOW and Phase 2 CAPTIVATE trials, fixed-duration combination of ibrutinib-venetoclax (I+V) resulted in deep and sustained clinical responses, and a broadly manageable safety profile in patients with CLL.
Several factors including genetic features, disease status, comorbidities, patient preferences, and safety profiles contribute to selection of the optimal therapeutic option for patients with CLL in the first-line setting. Adverse events (AEs), such as atrial fibrillation, bleeding, and hypertension, have been seen with the BTKi therapeutic class.Long-term clinical and real-world experience with ibrutinib has led to specific guidance regarding AE management, which has been included in the updated ibrutinib prescribing information (US PI, May 2022; EU SmPC, April 2023). Retrospective evidence, including post-hoc analyses of clinical trial data, demonstrates that dose reduction of ibrutinib mitigates recurrence or worsening of AEs while preserving efficacy. Thus, these findings together support the rationale to prospectively evaluate the impact of regimen and dosing flexibility of ibrutinib in patients with untreated CLL.
Aim: The key objective of the TAILOR study (NCT05963074) is to assess the efficacy and safety of I+V and ibrutinib monotherapy regimens with both a proactive and reactive dose adjustment in patients with untreated CLL.
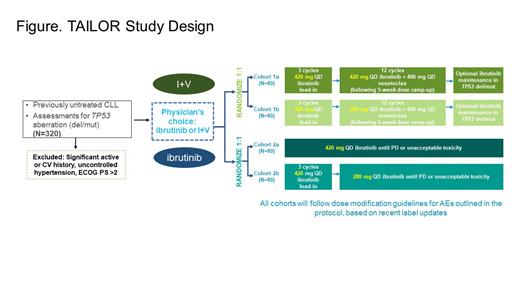
Methods: TAILOR is a phase 2, two-arm, four-cohort interventional study designed to provide physicians the choice of ibrutinib monotherapy or I+V combination therapy for previously untreated patients with CLL and prospectively evaluate proactive and reactive dose adjustment approaches. Eligible patients will have previously untreated active CLL, needing therapy as per iwCLL 2018 criteria. Key exclusion criteria include significant active or history of cardiovascular disease, uncontrolled hypertension, and ECOG performance status > 2. Patients will be randomized after selection of regimen: I+V fixed-duration regimen (2 cohorts, 80 patients in each cohort) or ibrutinib monotherapy (2 cohorts, 80 patients in each cohort), as per physician's choice. Patients in the I+V arm will receive ibrutinib 420 mg QD as lead-in for 3 cycles plus 12 cycles of either ibrutinib 420 mg QD (Cohort 1a) or 280 mg QD (Cohort 1b) in combination with venetoclax, which will be initiated in cycle 4 with dose ramp-up per label over 5 weeks (20, 50, 100, 200, and 400 mg/day) and continued at 400 mg/day dose from cycle 5 onward. In addition, patients with deletion 17p and/or mutated TP53 will receive optional ibrutinib maintenance. Patients in the ibrutinib monotherapy arm will receive ibrutinib 420 mg QD (Cohort 2a) or 420 mg QD for 3 cycles, then 280 mg QD (Cohort 2b) until progressive disease or unacceptable toxicity ( Figure). All cohorts will follow the dose modification guidance as per protocol based on the US PI/ EU SmPC. The primary endpoint is best overall response rate as assessed by investigators and compared with historic controls. Secondary endpoints include: complete response rate, duration of response, PFS, and OS; undetectable minimal residual disease rate (I+V cohorts only); safety (AEs, discontinuation due to AEs, adherence rates); and patient reported outcomes. The study is currently recruiting patients.
Disclosures
Sharman:Seattle Genetics: Research Funding; AbbVie, AstraZeneca, BMS, Beigene, Lilly, Genentech, Inc., Genmab: Consultancy; Merck, Novartis: Consultancy; AbbVie, AstraZeneca, BeiGene, BMS, Genentech, Inc., Lilly: Consultancy. Burger:Abbvie, Beigene: Research Funding; Janssen: Other: Speaker fees and Travel Support; AstraZeneca, Pharmacyclics: Other: Advisory Board, Research Funding. Barrientos:Abbvie: Other: Advisory Board; Beigene: Honoraria, Other: Advisory Board; AstraZeneca: Honoraria; Merck: Other: Advisory Board, Research Funding. Kuruvilla:Janssen, AstraZeneca: Honoraria; Abbvie, Novartis, Sanofi: Other: Advisory Board; Janssen: Other: TAILOR Steering Committee. Patel:AbbVie: Current Employment, Divested equity in a private or publicly-traded company in the past 24 months. Krigsfeld:Abbvie: Current Employment, Current holder of stock options in a privately-held company, Divested equity in a private or publicly-traded company in the past 24 months. Srikanthan:Janssen: Current Employment. Messahel:Janssen: Current Employment. Khan:Janssen: Current Employment. Smith:Janssen: Current Employment. Ghia:Janssen: Consultancy, Honoraria, Research Funding; MSD: Consultancy, Honoraria, Research Funding; Lilly/Loxo Oncology: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal